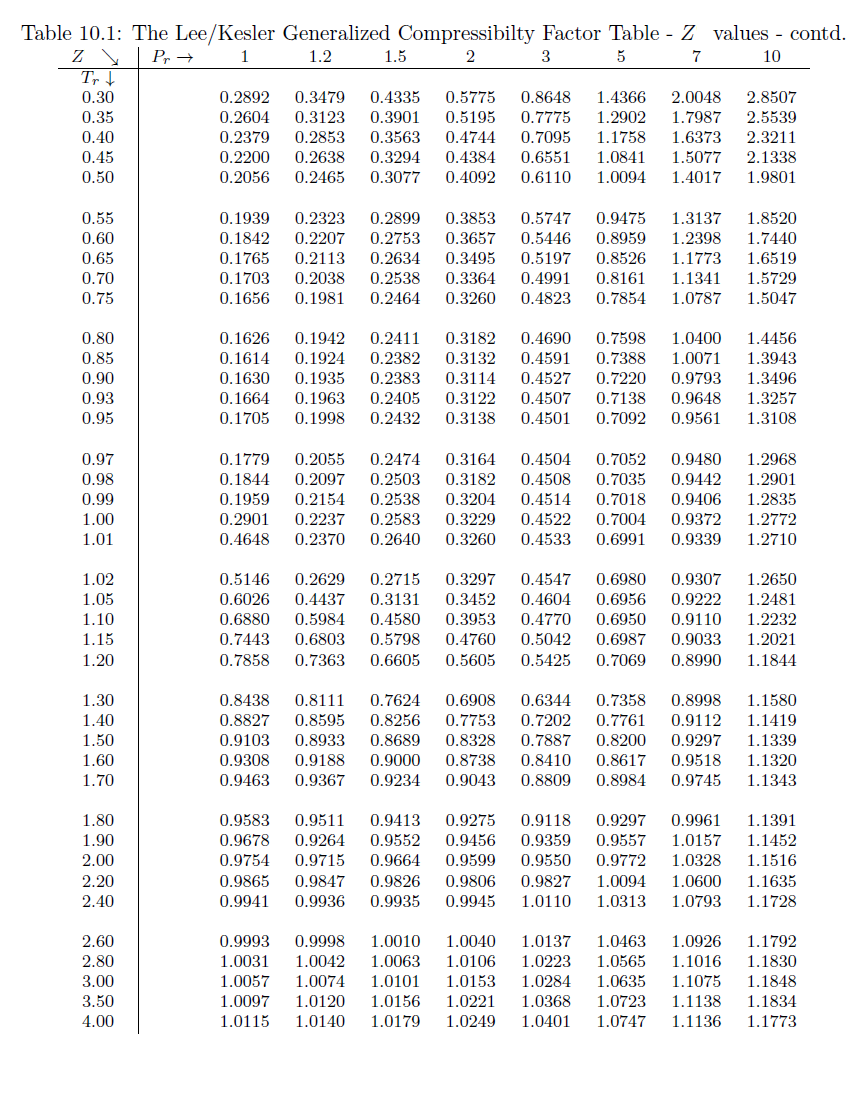
physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
5 (702) · $ 28.99 · In stock

The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In
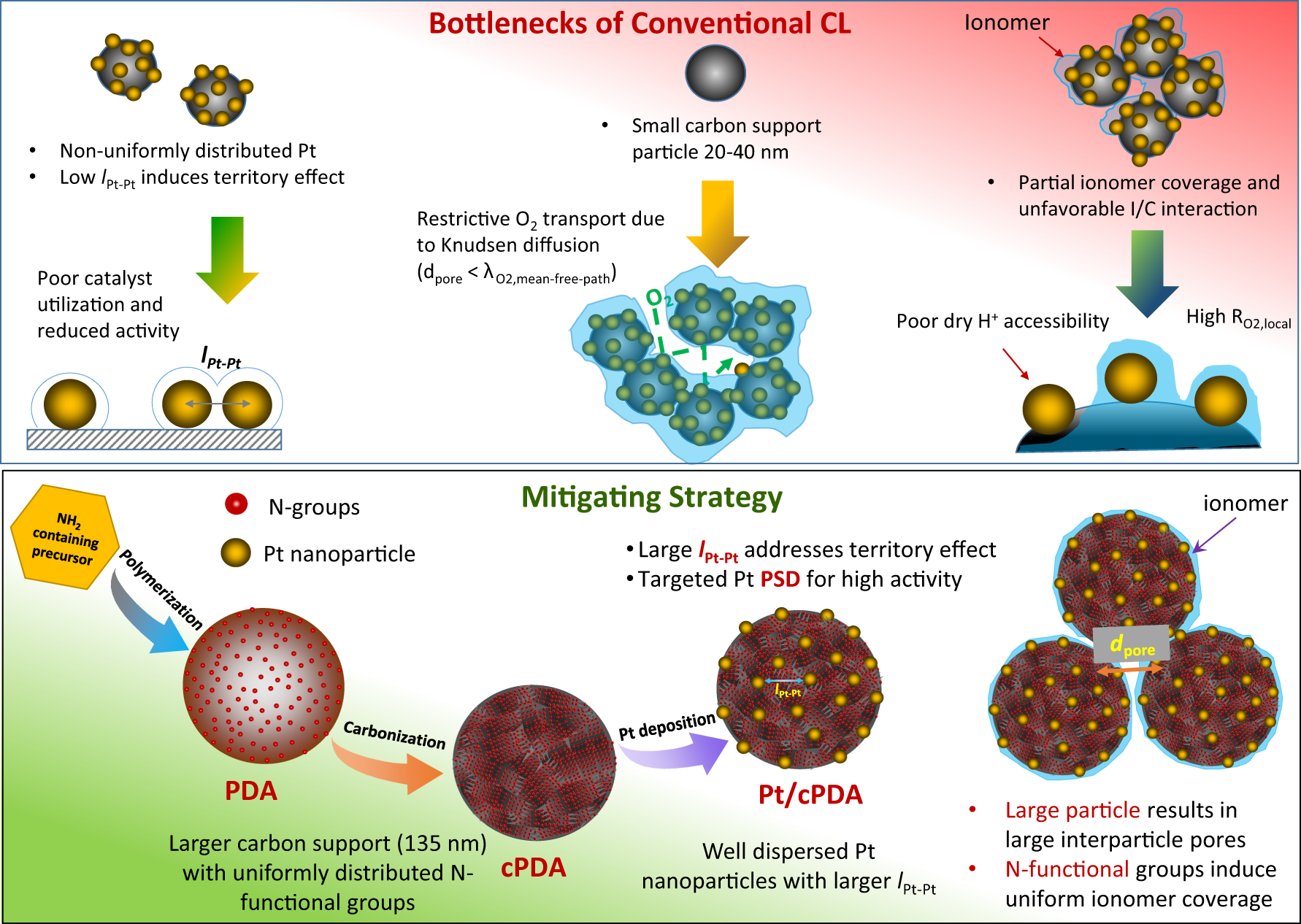
Designing fuel cell catalyst support for superior catalytic
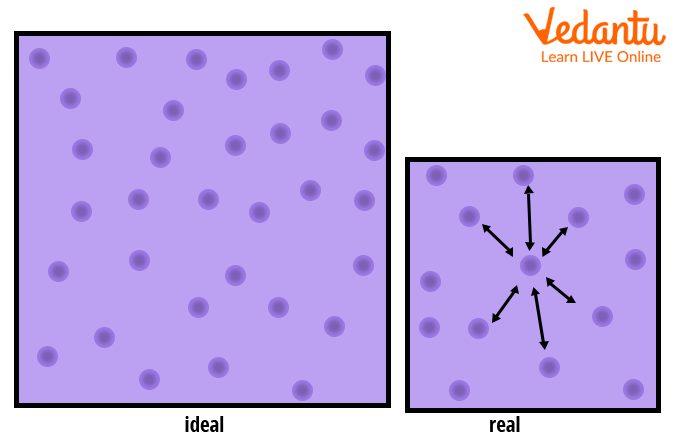
JEE - Compressibility Factor Important Concepts and Tips
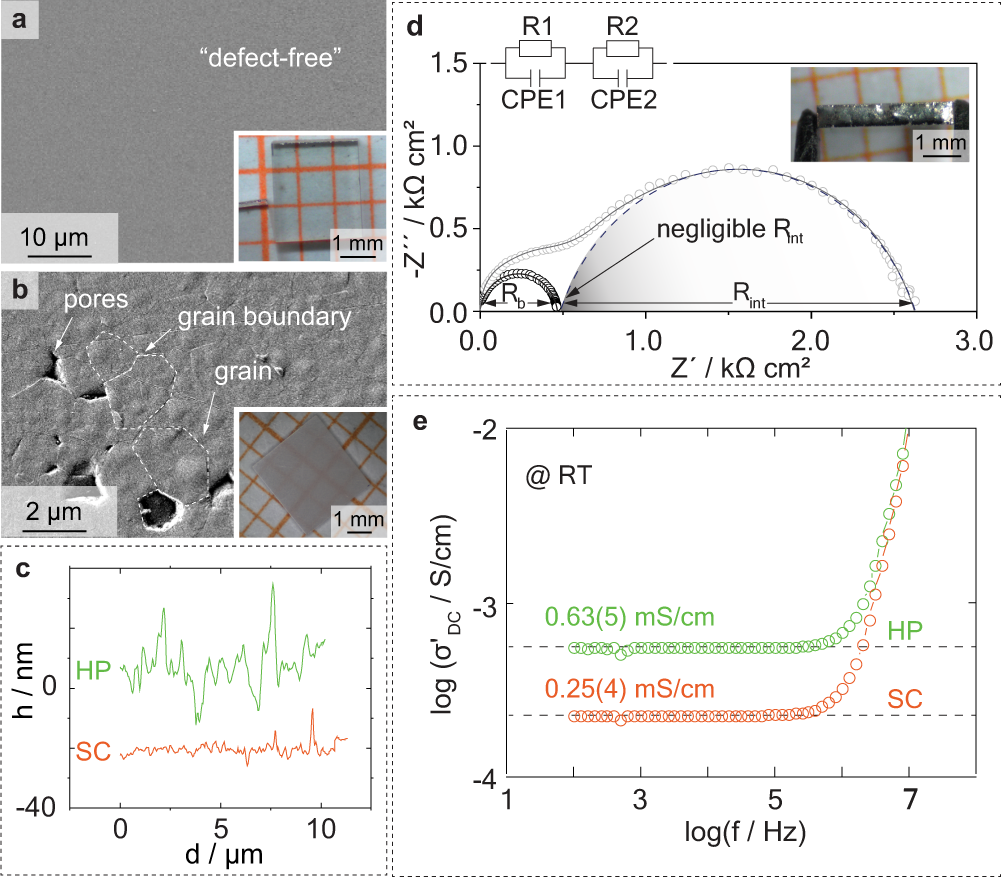
Effect of pulse-current-based protocols on the lithium dendrite
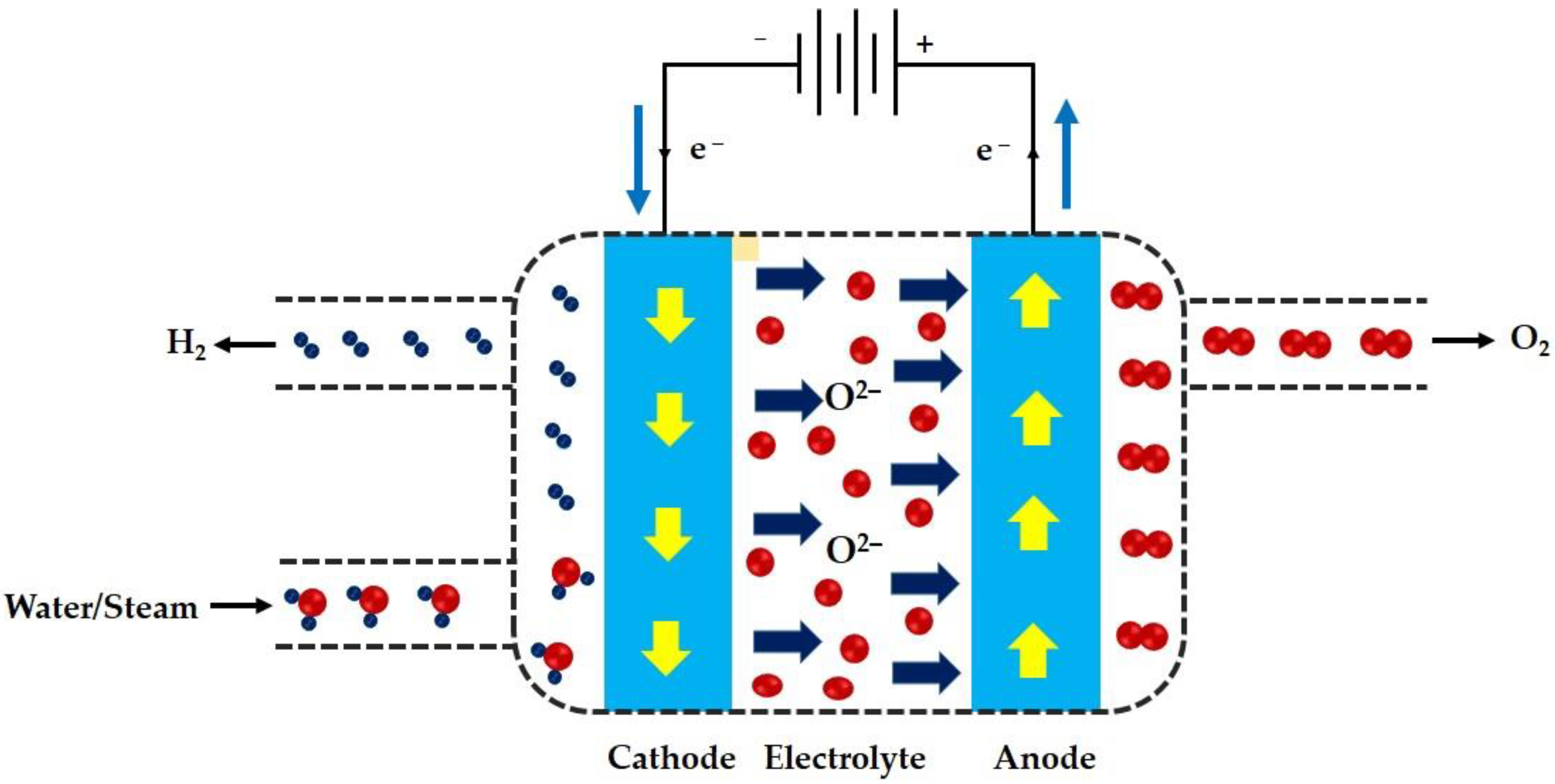
Energies, Free Full-Text

Computational Chemistry as Applied in Environmental Research
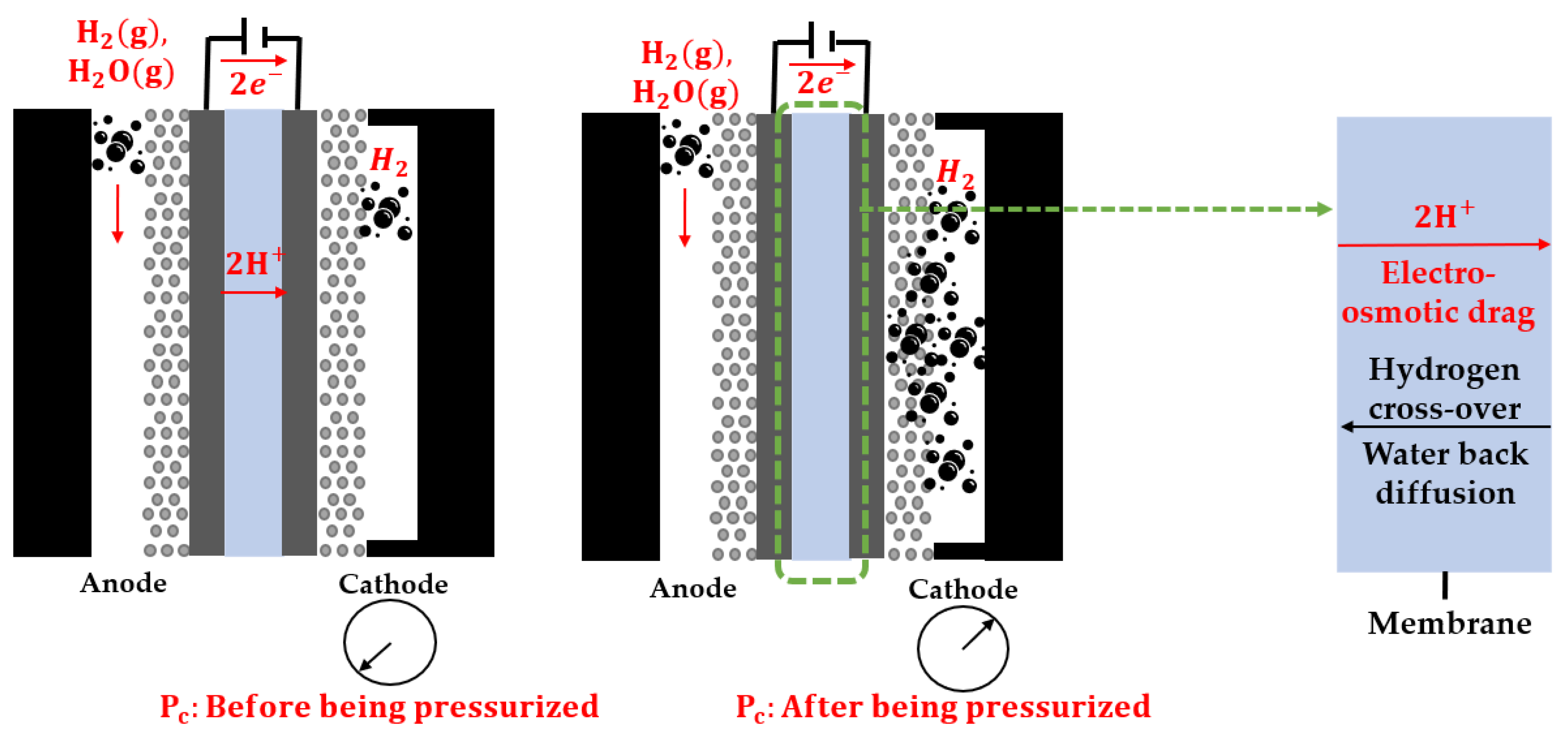
Membranes, Free Full-Text

Effects of mechanical pressure on anion exchange membrane water

temperature - In a liquid-in-glass thermometer, how does the gas

822 questions with answers in PHYSICAL CHEMISTRY