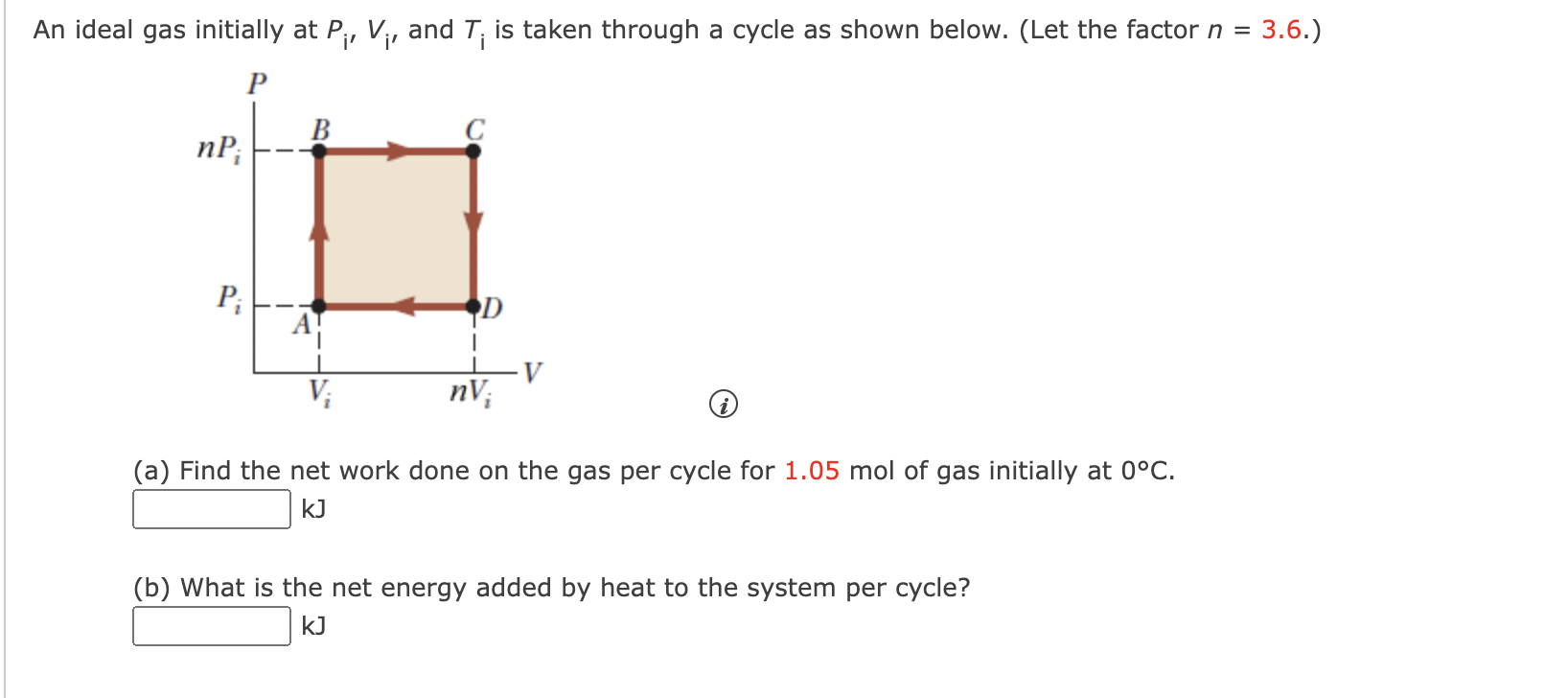
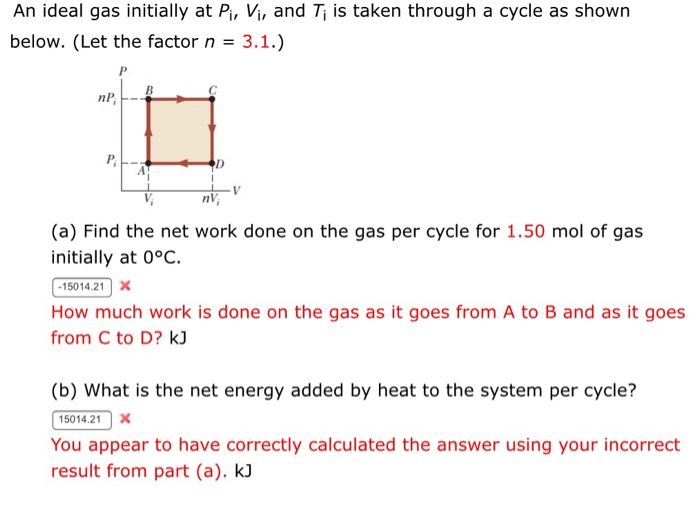
Solved An ideal gas initially at Pi, Vi, and Ti is taken
4.7 (613) · $ 13.00 · In stock

One mole of a monoatomic ideal gas initially at a pressure of 2.00 bar and a temperature of 273 K is

SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n 3.6.) nP; P; nV; (a Find the net work done

Molar Mass & Ideal Gas Law, Overview, Formula & Examples - Lesson
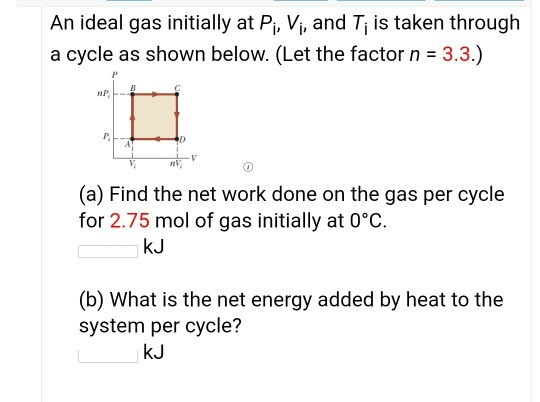
Solved An ideal gas initially at Pi, Vi, and Ti is taken
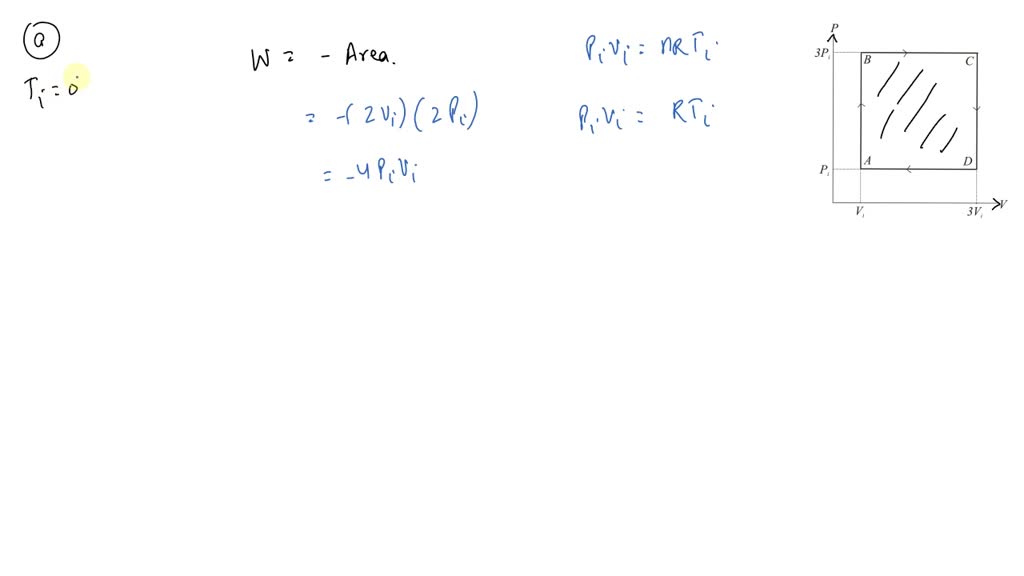
⏩SOLVED:An ideal gas initially at Pi, Vi and Ti is taken through a…

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of
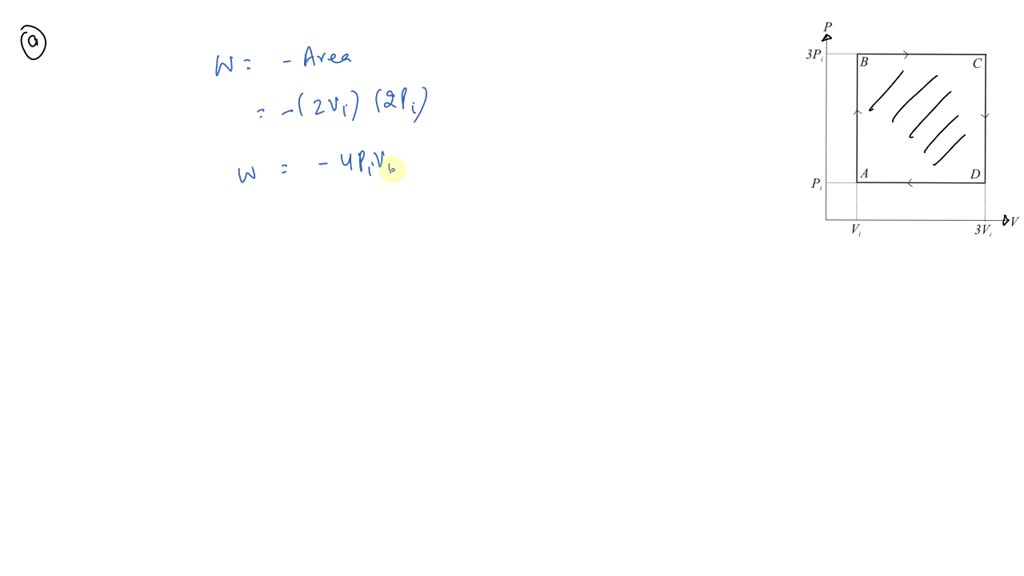
⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…

Combined Gas Law — Overview & Calculations - Expii
![2024] 200+ Chemistry Courses to Expand Your Knowledge of the World — Class Central](https://www.classcentral.com/report/wp-content/uploads/2021/12/chemistry-courses-2021-12-7.png)
2024] 200+ Chemistry Courses to Expand Your Knowledge of the World — Class Central

⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…

SOLVED: Diatomic ideal gas (γ = 1.40) confined to a cylinder through a closed cycle. Initially, the gas is at Pi, Vir, and Ti. First, its pressure doubles under constant volume. It