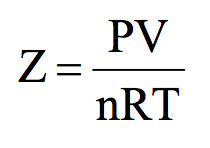The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
4.5 (781) · $ 16.99 · In stock
The compression factor (compressibility factor) for one mole of a van der Waals

States Of Matter Notes: Class 11, JEE, NEET, AIIMS
For one mole real gas, the correct value of Z at point P using following graph is - Sarthaks eConnect
For 1 mol of gas, the plot of pV vs p is shown below. p is the pressure and V is the volume of the gas. - Sarthaks eConnect

The graph of compressibility factor (Z) v/s P 1 mol of a real gas is shown in following diagram. The graph is plotted 273 K temperature. If slope of graph very high

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

Solved We begin by showing that the compressibility factor
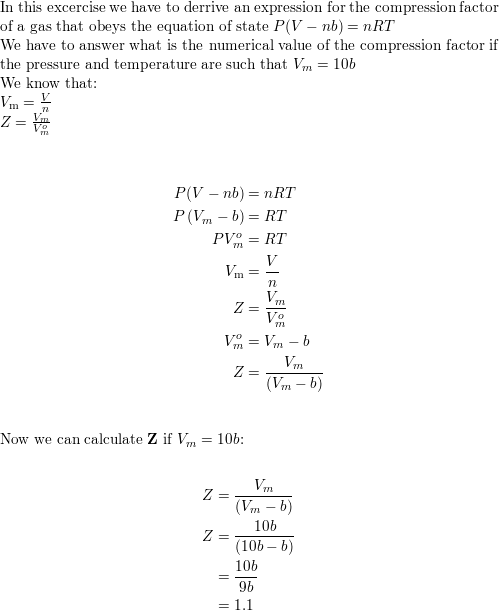
Derive an expression for the compression factor of a gas tha
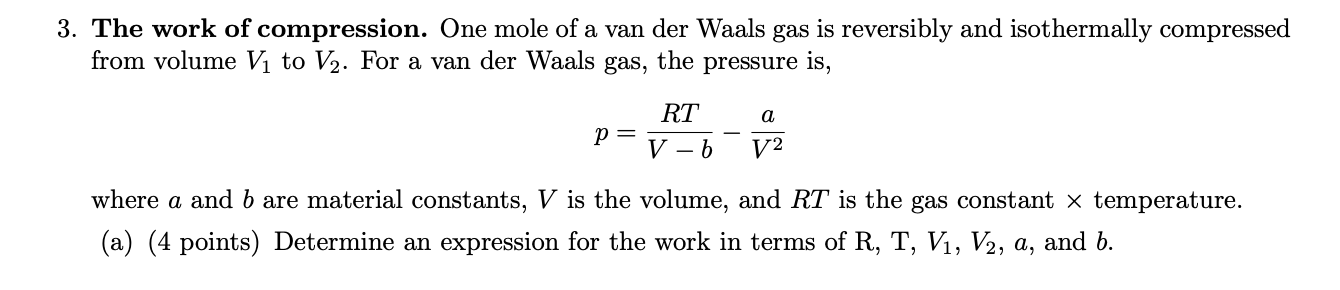
Solved 3. The work of compression. One mole of a van der
The value of compression factor at the critical state of a vander waals gas is

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

Solved We showed, for a van der Waals gas, that the