What is the change in internal energy (in J) of a system that
5 (359) · $ 19.00 · In stock

I found an increase of 3100J Have a look

What is the change in internal energy (in J) of a system that

Answered: A system does 596 kJ of work and loses…

Solved What is the change in internal energy (in J) of a

If change in internal energy -80 J. The work done by system is +40 J.

Answered: 1. What is the change in internal…

Ch6.1 The Nature of Energy (hustle!) - ppt download
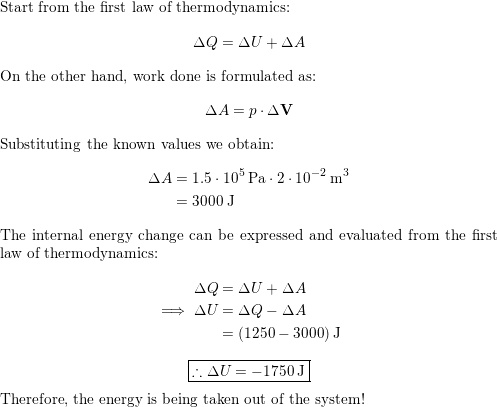
A chemical reaction transfers 1 250 J of thermal energy into

15.4 What is the change in internal energy of a system which
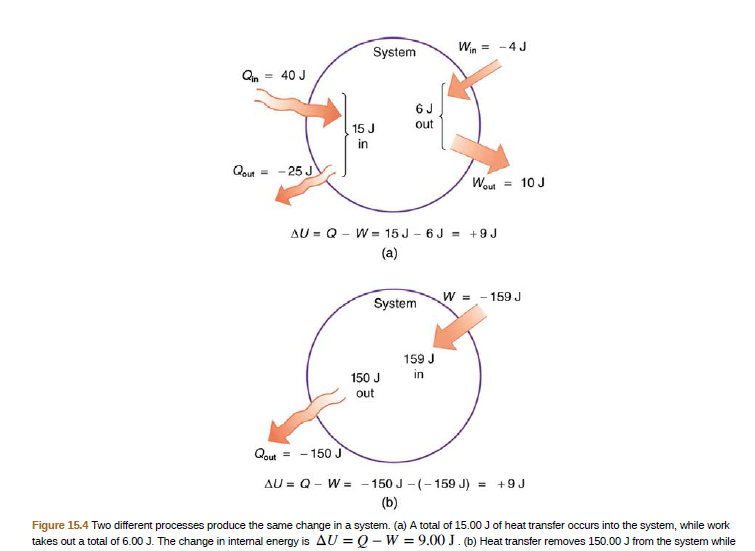
Answered: System Win = -4J Qn = 40 J 15 J out in…
What is the change in internal energy of a system that absorbs 455 J of heat and does 325 J of work? - Quora

Calculate the change in internal energy delta E for a system that is giving off 25 0kJ of heat and i

Solved What is the change in internal energy in J) of a

Solved Be sure to answer all parts. What is the change in

For a system that has equally spaced non-degenerate energy levels
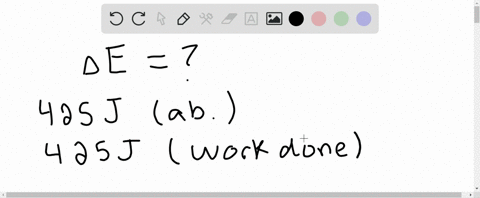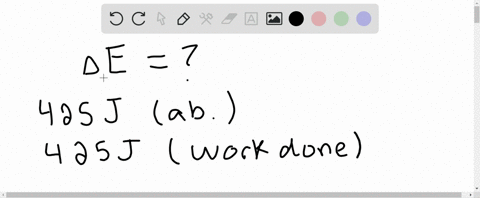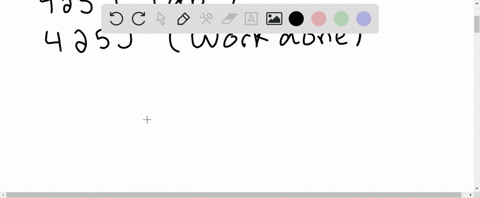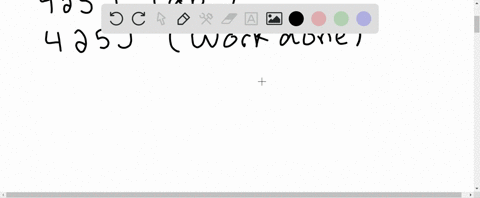
⏩SOLVED:A system receives 425 J of heat from and delivers 425 J







