Solved A 45-g block of copper at −12∘C is added to 120 g of
4.8 (259) · $ 22.50 · In stock
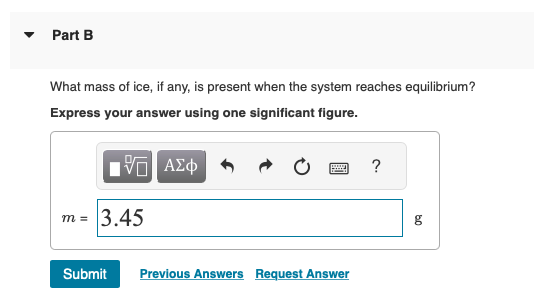
Answer to Solved A 45-g block of copper at −12∘C is added to 120 g of
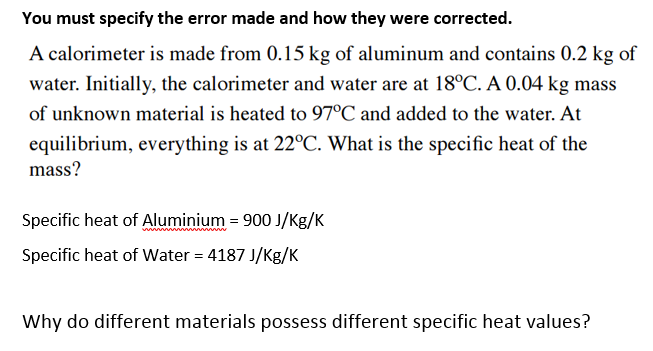
Answered: A calorimeter is made from 0.15 kg of…

Specific Heat Capacity
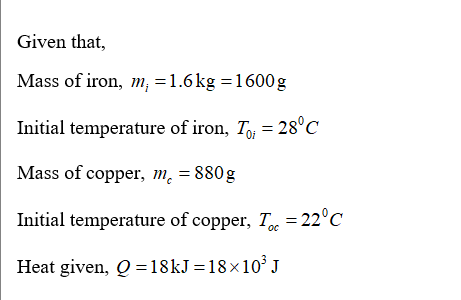
Answered: A1.6 kg block of iron at 28 Cis…

Solved 3) A sample of copper was heated to 120 °C and then

A thermally insulated, closed copper vessel contains water at 15^@C. W
✓ Solved: The heat capacity of a bomb calorimeter was determined by burning 6.79 g of methane (energy

A steel pipe with a 12 inch outer diameter is fabricated from one 4 inch thick plate by welding along a helix which forms an angle of 45 degrees with a plane

Solved A 45-g block of copper at −12∘C is added to 120 g of
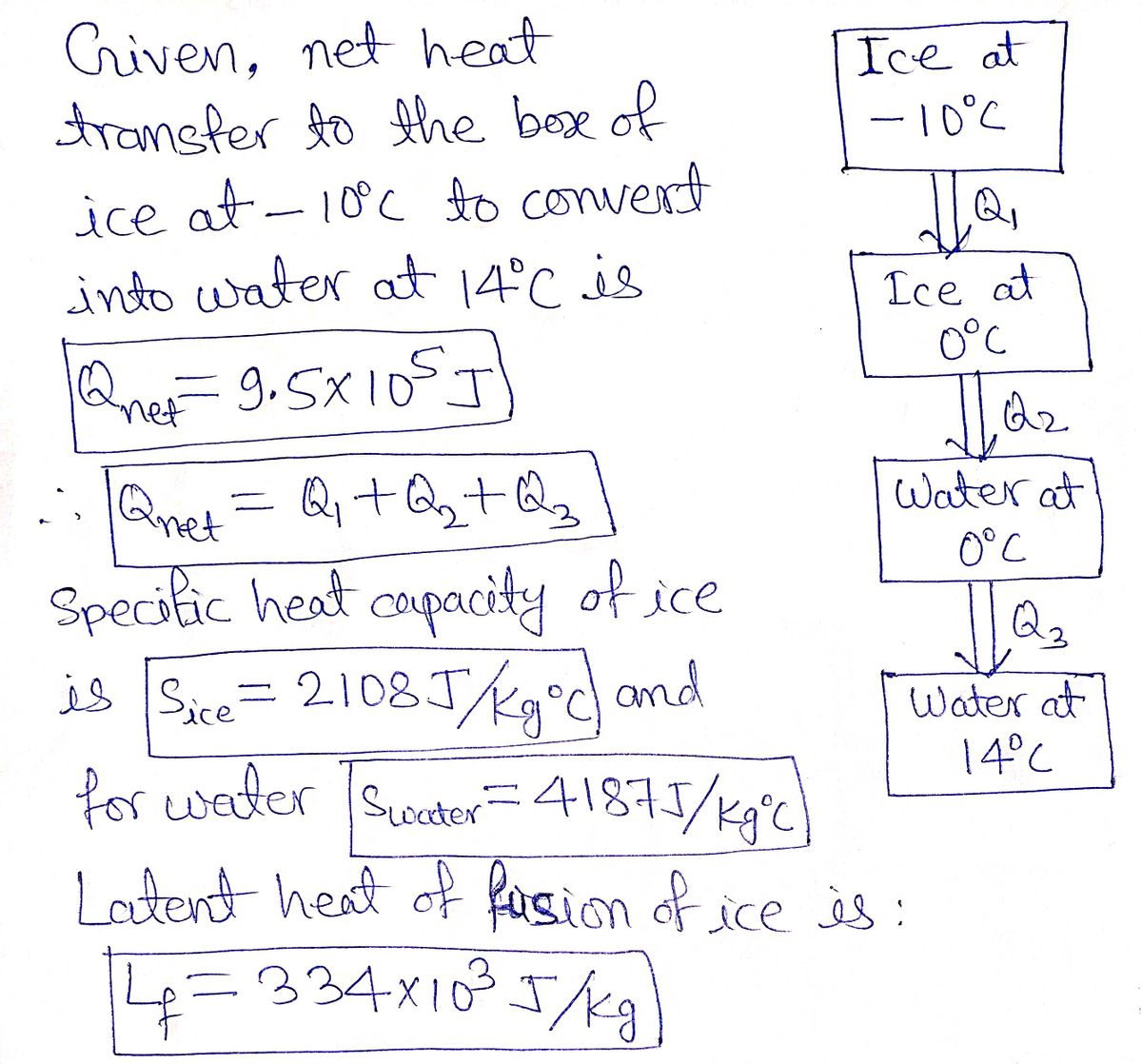
Answered: A heat transfer of 9.5×105 J is…

Specific Heat Capacity

Temperature Change and Heat Capacity
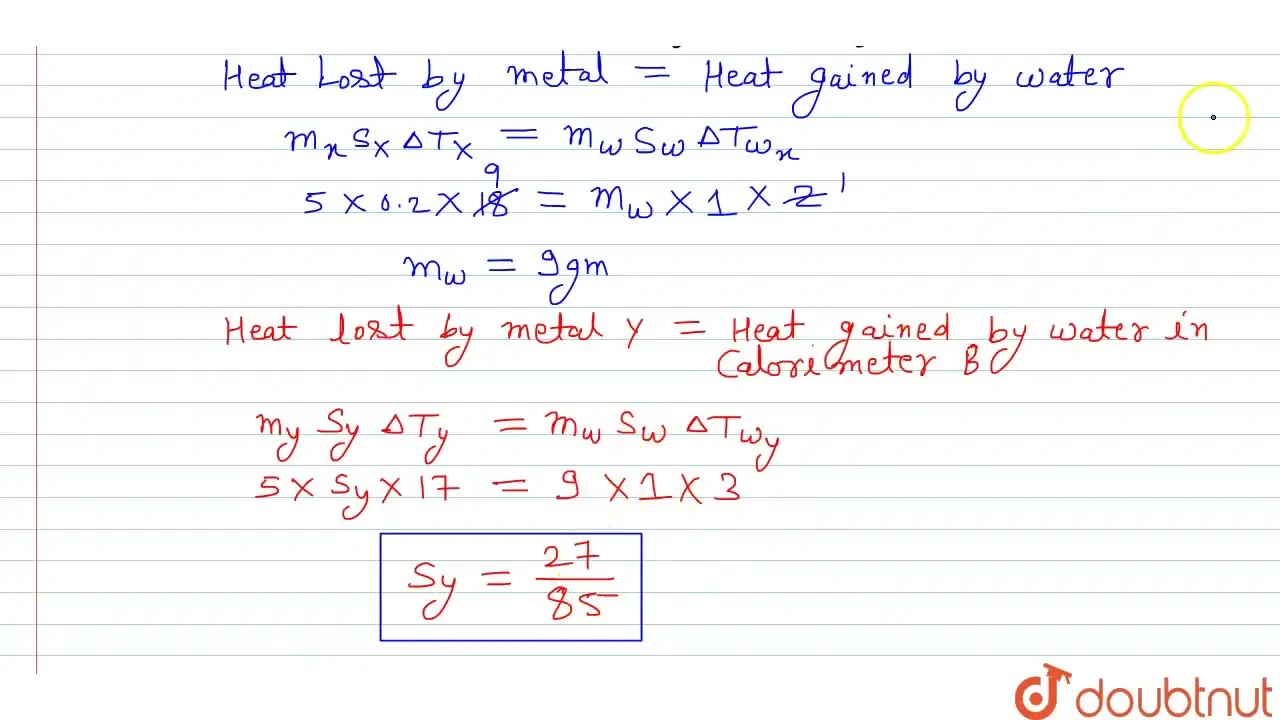
Two identical calorimeters A and B contain an equal quantity of water

200 g of hot water at 80^@C is added to 300 g of cold water at 10^@C.

⏩SOLVED:A 45.0 g block of tungsten at 30.0^∘ C and a 25.0 g block…
2 kg of ice at 0°c is mixed with 8 kg of water at 20°c. What is the final temperature? - Quora







